
body contouring technology
by surgeons¹ and is reshaping
the way we feel, see and
experience results.


contouring technology by surgeons¹
and is reshaping the way we feel, see
and experience results.

PHYSICIAN FINDER
Enter your Name, Email and Zip Code below to find physicians near you that use Renuvion.
Let’s Talk
Loose Skin
In fact, many people experience unwanted loose skin after undergoing liposuction. While liposuction is effective at removing stubborn fat, it doesn't address the loose skin that often results from aging or weight loss. In some cases, the reduction in fat volume can actually worsen skin laxity. That’s where Renuvion fits in.
New mom? Weight loss? Aging? Loose skin happens and is caused by the breakdown of collagen-rich tissue, which is one of the key factors to contributing to a loss of skin elasticity.
In fact, many people experience unwanted loose skin after undergoing liposuction. While liposuction is effective at removing stubborn fat, it doesn't address the loose skin that often results from aging or weight loss. In some cases, the reduction in fat volume can actually worsen skin laxity. That’s where Renuvion fits in.
We Have A Solution
Renuvion is the #1 trusted body contouring technology by surgeons¹ and designed to treat loose skin directly at the source. Here’s how: The Renuvion wand is inserted under the skin, using a unique combination of radiofrequency energy and helium plasma to target that collagen-rich tissue. This energy contracts those collagen fibers, pulling the skin closer to the muscle for a smooth, contoured appearance in just minutes.
Real patients,
real results
Since 2017, over 325,000 people have experienced Renuvion’s transformative results². It delivers durable and long-lasting results allowing patients to look in the mirror and see a renewed version of themselves.
Let’s Talk
Loose Skin
New mom? Weight loss? Aging? Loose skin happens and is caused by the breakdown of collagen-rich tissue, which is one of the key factors to contributing to a loss of skin elasticity.
In fact, many people experience unwanted loose skin after undergoing liposuction. While liposuction is effective at removing stubborn fat, it doesn't address the loose skin that often results from aging or weight loss. In some cases, the reduction in fat volume can actually worsen skin laxity. That’s where Renuvion fits in.
We Have A Solution
Renuvion is the #1 trusted body contouring technology by surgeons1 and designed to treat loose skin directly at the source. Here’s how: The Renuvion wand is inserted under the skin, using a unique combination of radiofrequency energy and helium plasma to target that collagen-rich tissue. This energy contracts those collagen fibers, pulling the skin closer to the muscle for a smooth, contoured appearance in just minutes.
Real patients,
real results
Since 2017, over 325,000 people have experienced Renuvion’s transformative results2. It delivers durable and long-lasting results allowing patients to look in the mirror and see a renewed version of themselves.
Targeting loose skin directly at the source
Here's how we do it.
CONTOURING
The Renuvion Body
Renuvion is the only device that is FDA‑cleared for use after liposuction, and the only device FDA‑cleared for contracting subcutaneous soft tissue.
HOW IT WORKS
The wand is inserted under the skin and delivers a unique combination of radiofrequency energy and helium plasma to the collagen-rich tissue that’s a primary cause of loose skin. This energy contracts these collagen fibers and pulls the skin down, closer to the muscle, for a more contoured appearance. Renuvion is the result of decades of research, evidence-based medicine and clinical data.
REAL RESULTS
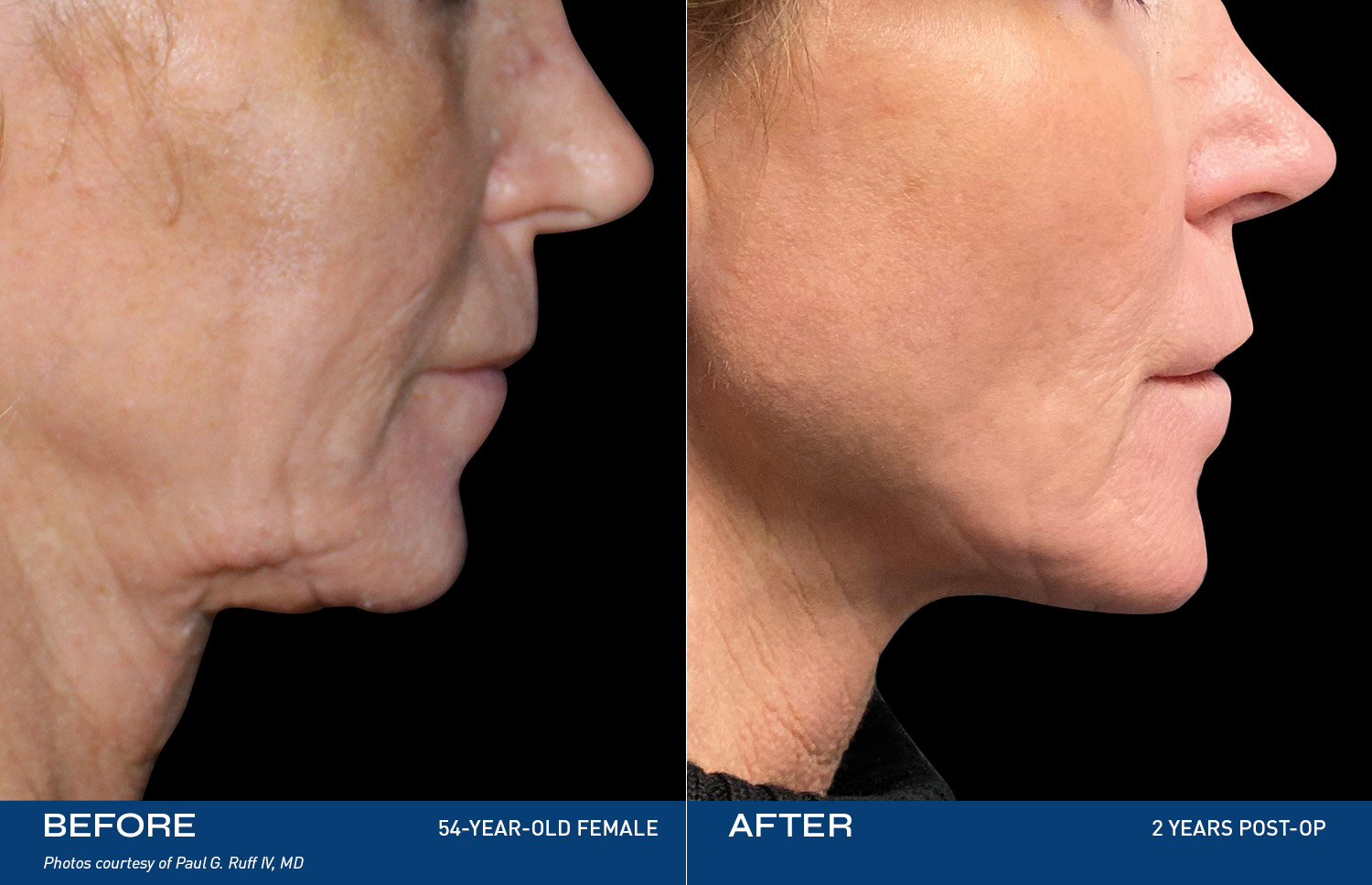
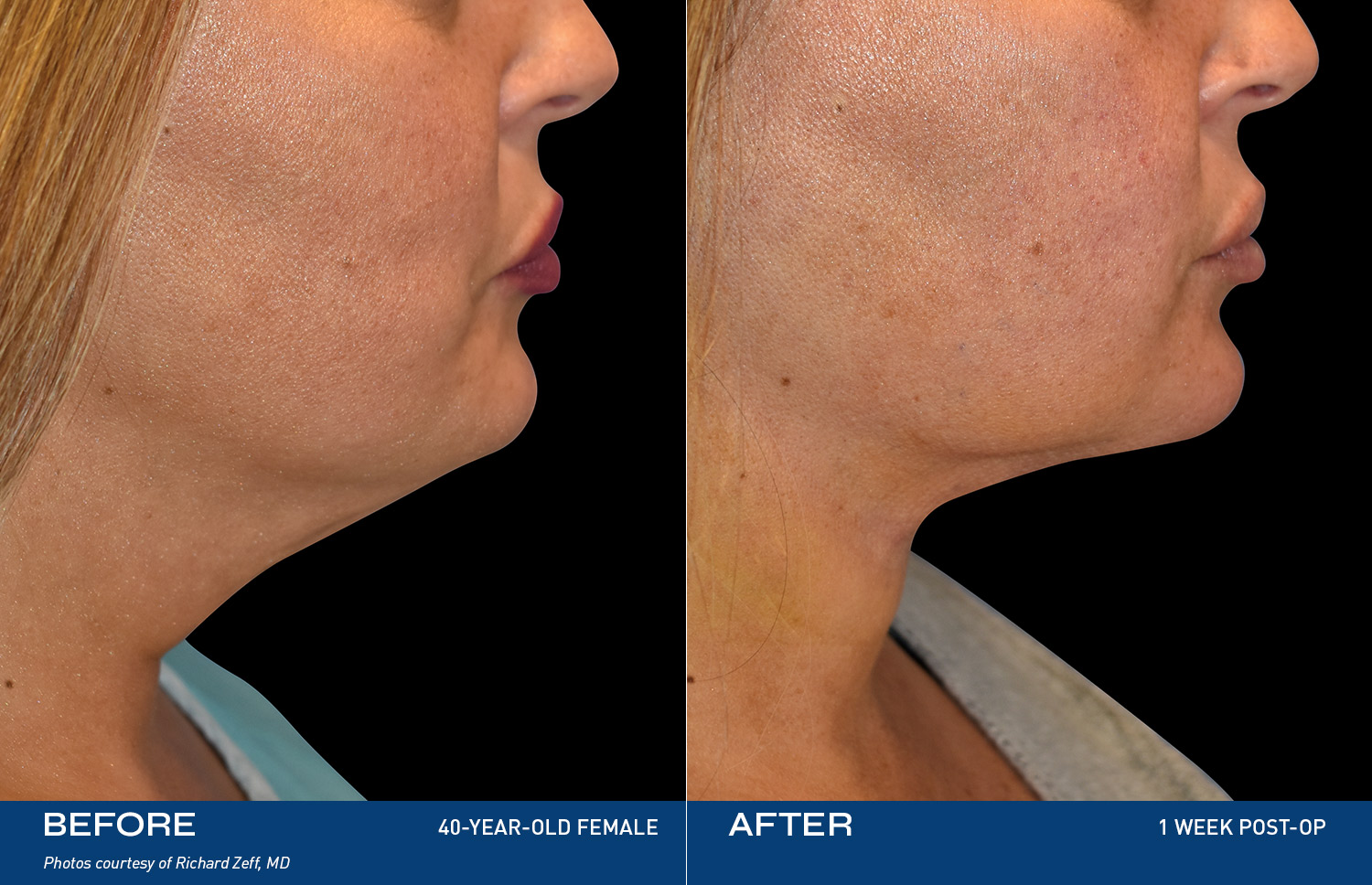
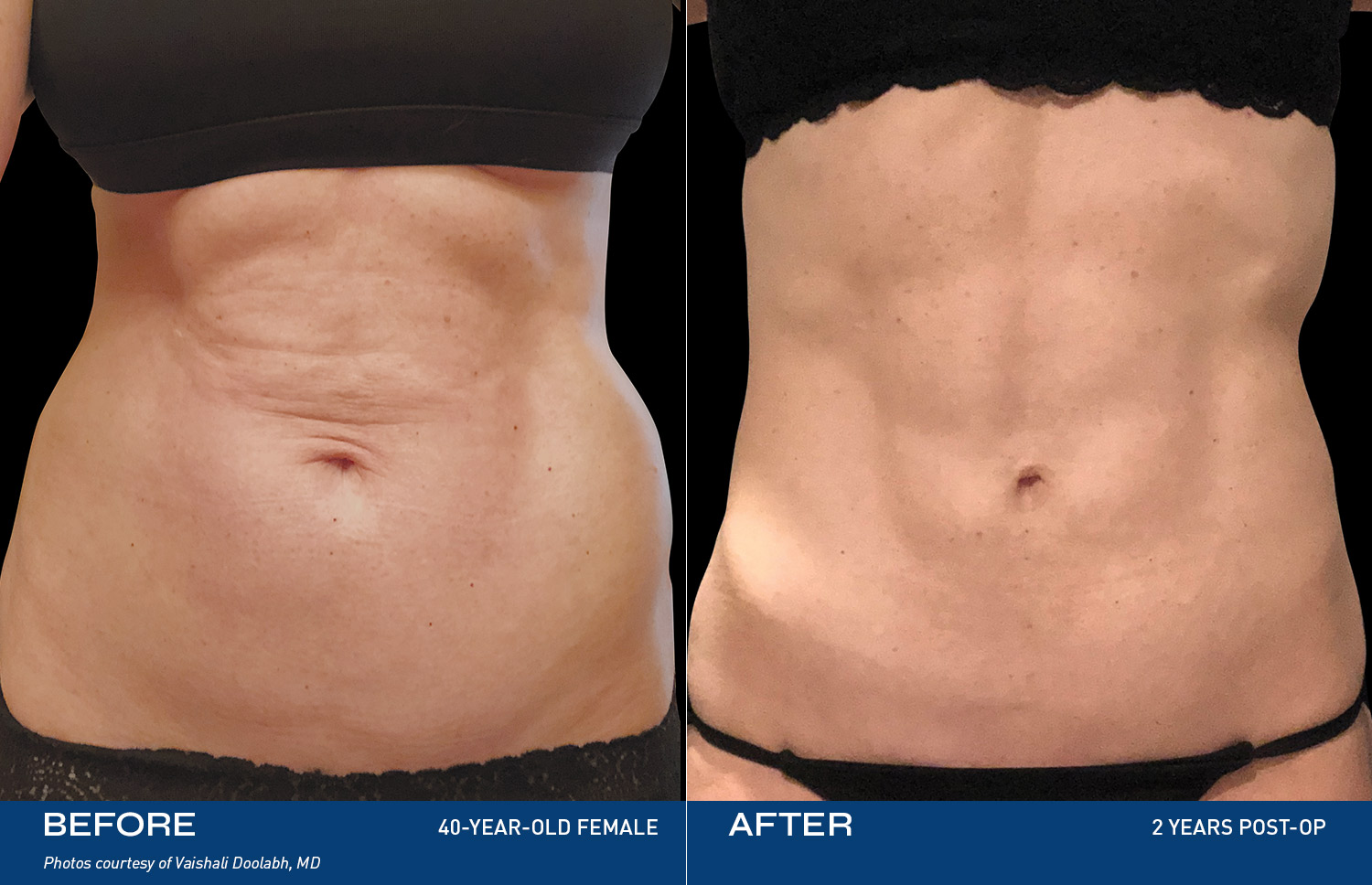
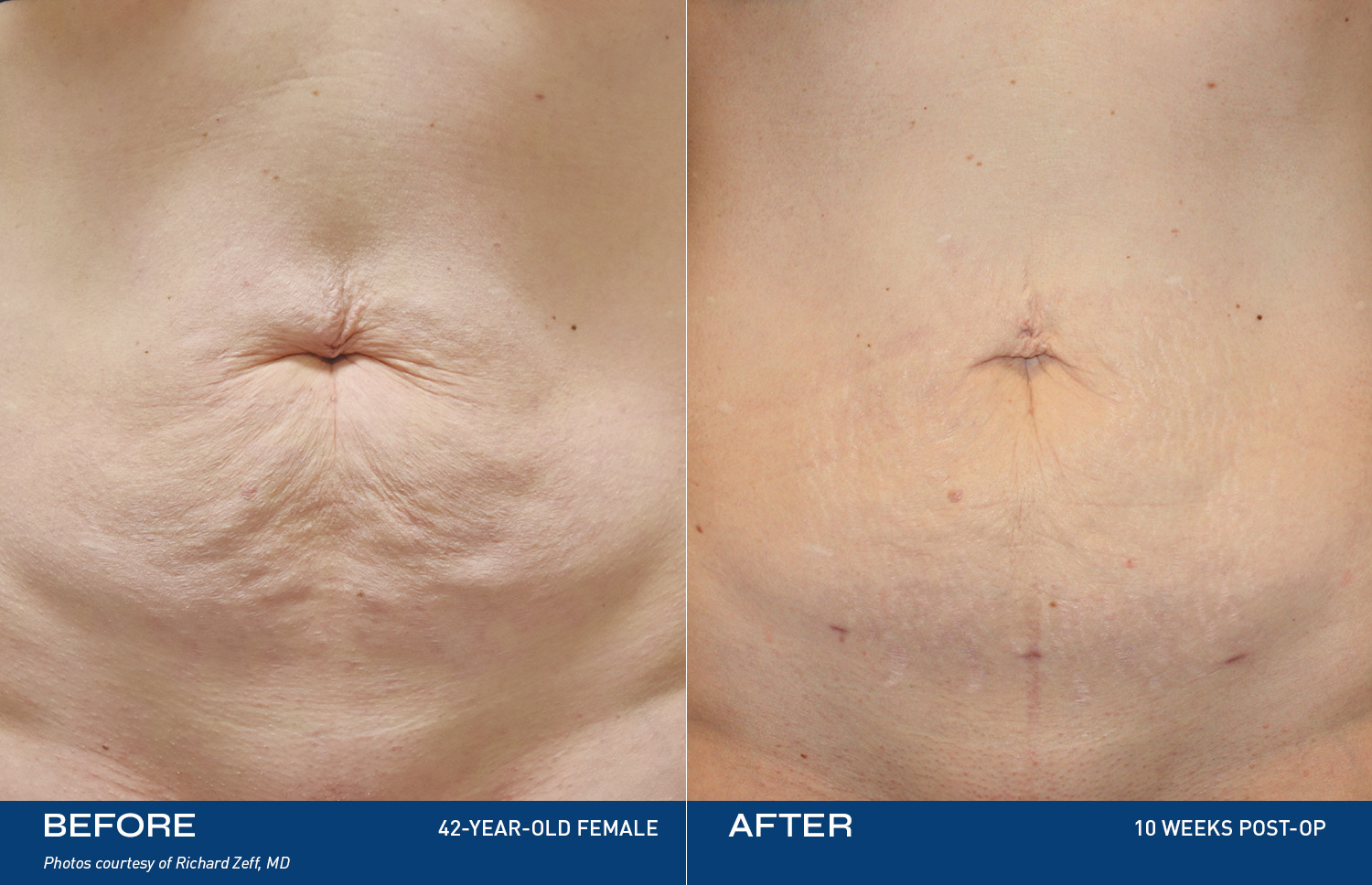
BEFORE & AFTERS
“I have always been self-conscious of my chin & neck area and was ready to finally do something about it. Renuvion completely changed the way I look at myself in the mirror. I finally feel like the best version of myself and can proudly say, this is ME."
- Renuvion Patient
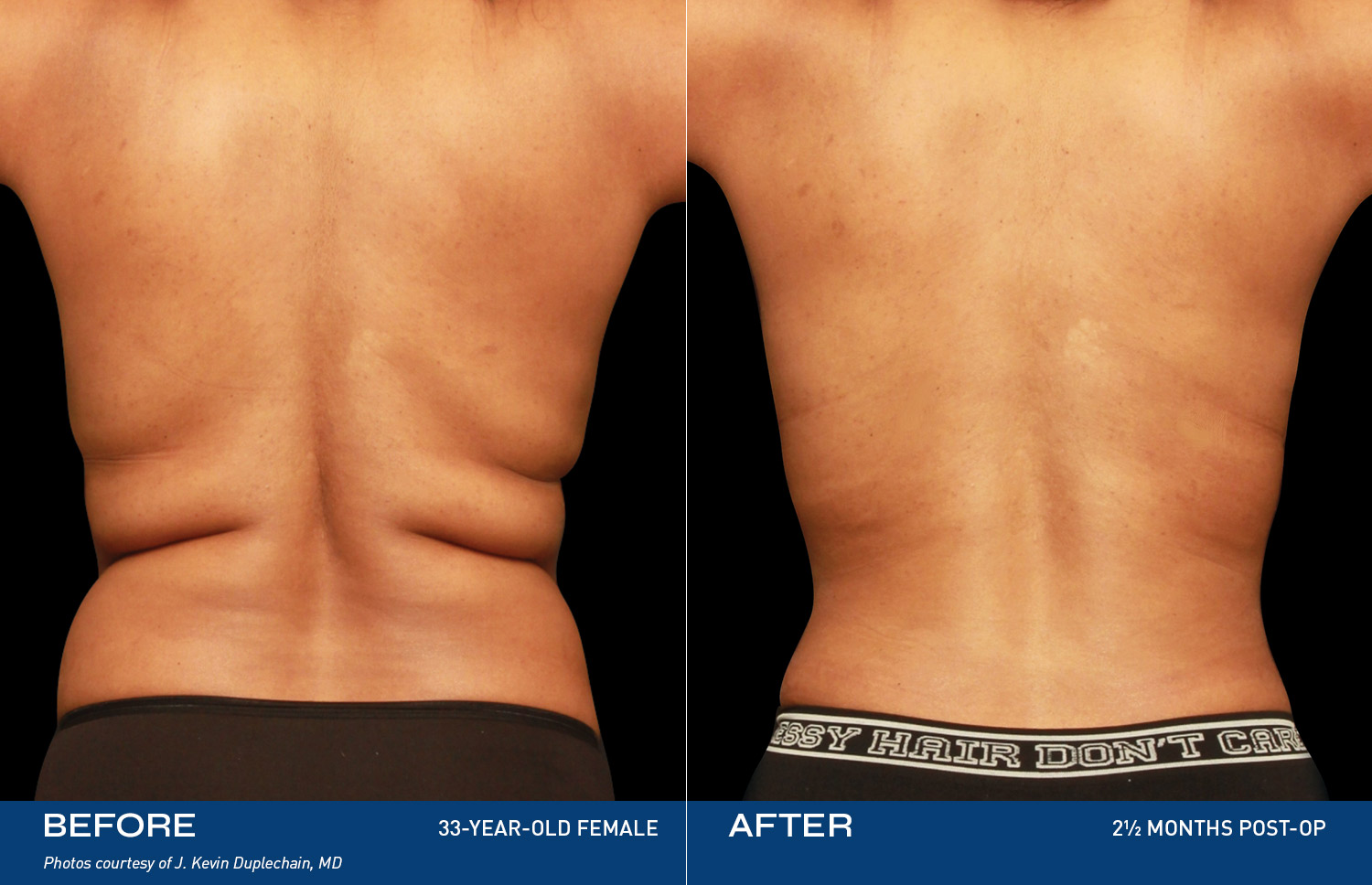
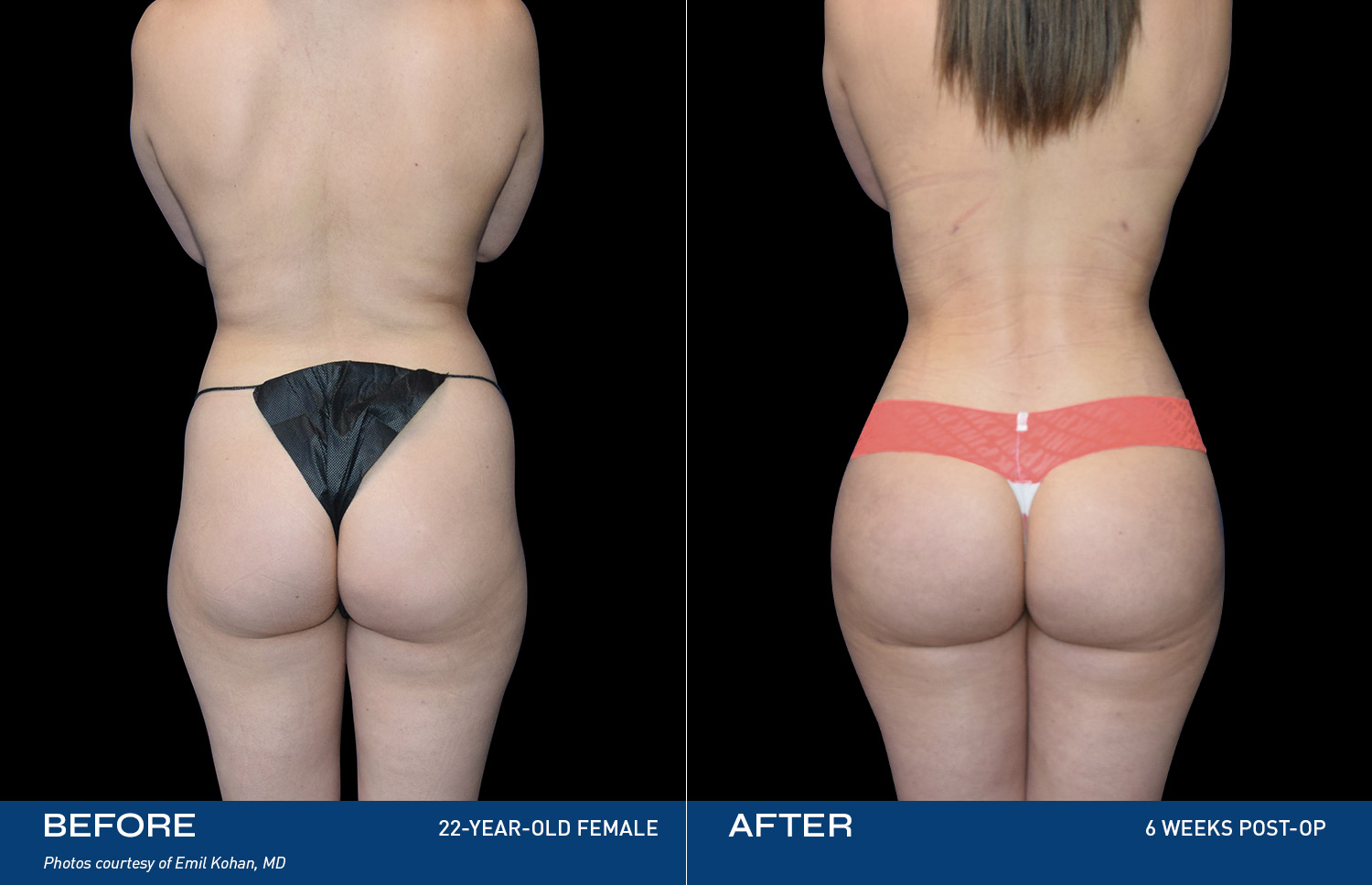
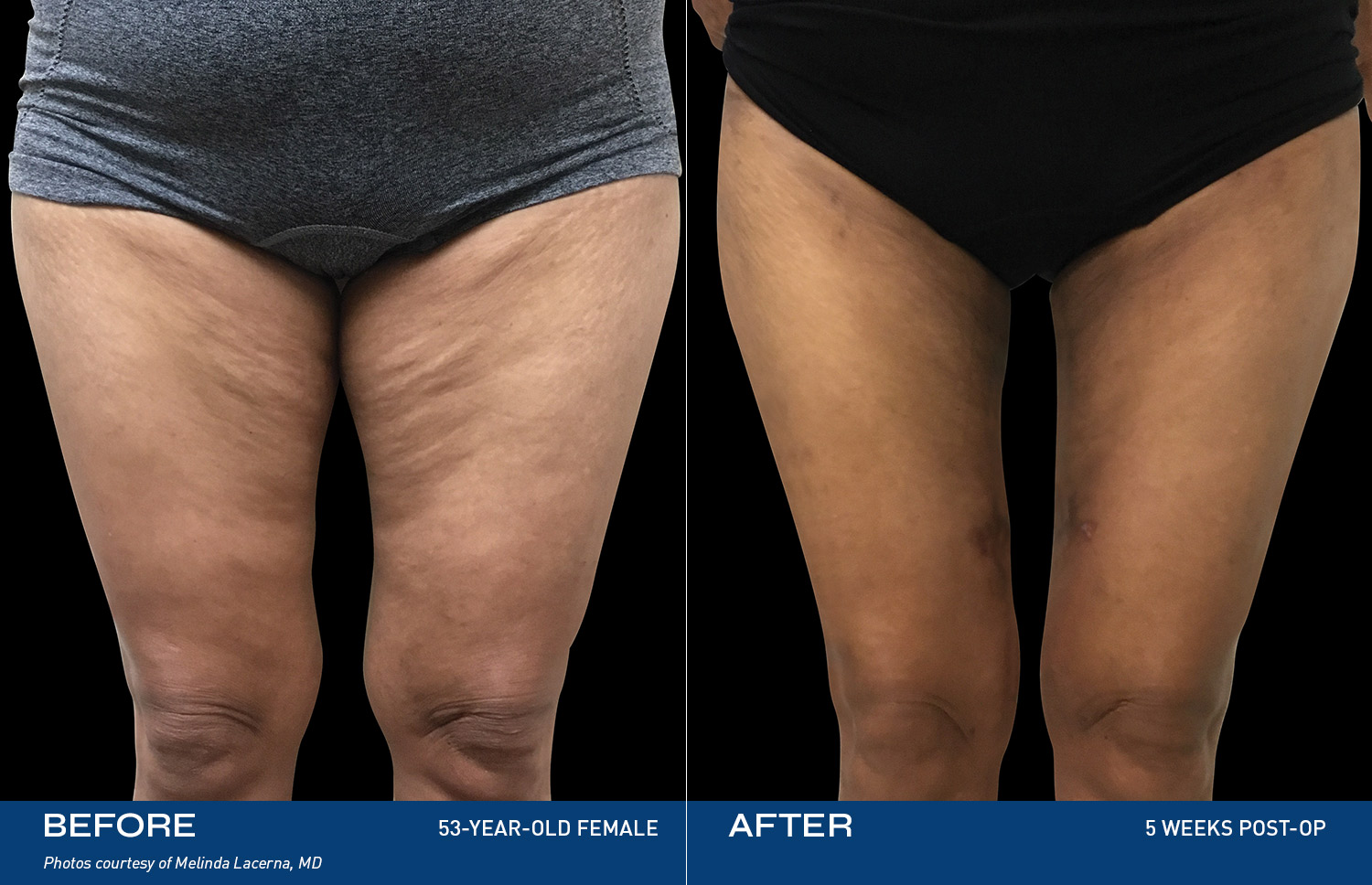
What patients are saying
Megan
Sloane
Keli
doctor
PAUL RUFF
doctor
Alexis Delobaux
doctor
Gregory Buford
FREQUENTLY ASKED QUESTIONS
Renuvion adds about 15 minutes per treated area to a standard liposuction procedure, though this can vary by patient.
Renuvion can be performed under both general and local anesthesia. It is always best to discuss these treatment options with your physician.
Depending on the number of areas being treated and your discussed anesthesia plan, Renuvion for Body Contouring recovery can be from a few days to a few weeks. Like any procedure, your treatment plan may result in a different level of recovery than someone else’s. Recovery expectations should always be discussed with your provider depending on your unique needs.
The duration of results varies for each individual. We have seen patients remain satisfied with their Renuvion results for up to 8 years, though it can take 6 to 9 months for the full results to become fully apparent.2,3
LATEST NEWS
AS SEEN IN











The Renuvion APR Handpiece is intended for the delivery of radiofrequency energy and/or helium plasma where coagulation/contraction of soft tissue is needed. Soft tissue includes subcutaneous tissue. The Renuvion APR Handpiece is intended for the coagulation of subcutaneous soft tissues following liposuction for aesthetic body contouring. The Renuvion APR Handpiece is indicated for use in subcutaneous dermatological and aesthetic procedures to improve the appearance of lax (loose) skin in the neck and submental region. The Renuvion APR Handpiece is intended for the delivery of radiofrequency energy and/or helium plasma for cutting, coagulation and ablation of soft tissue during open surgical procedures. The Renuvion APR Handpiece is intended to be used with compatible electrosurgical generators owned by Apyx® Medical. Not all indications are approved in all markets; check with your local sales representative for further information.
As with any aesthetic procedure, individual results may vary. As with all energy devices there are inherent risks associated with its use. Risk associated with the use of the Renuvion APR may include: helium embolism into the surgical site due to inadvertent introduction into the venous or arterial blood supply system, unintended burns (deep or superficial), pneumothorax, temporary or permanent nerve injury, ischemia, fibrosis, infection, pain, discomfort, gas buildup resulting in temporary and transient crepitus or pain, bleeding, hematoma, seroma, subcutaneous induration, pigmentation changes, increased healing time, scarring, asymmetry and/ or unacceptable cosmetic result. Please see the instructions for use for more detailed information.
References:
1. 4 out of 5 surgeons agree Renuvion is the #1 trusted body contouring technology. Results based on a 2024 Renuvion Physician survey conducted by Wakefield Research. Data on File.
2. Based on HP data on file.
3. FDA 510(k) Premarket Notifications K230272 & K223262.
4. Body contouring is a broad term that includes many technologies and surgical techniques. Body contouring procedures are intended to provide aesthetic reshaping rather than a change in physical weight. Skin laxity is a type of body contouring according the American Board of Cosmetic Surgery. Skin laxity is the quality of looseness or drooping in the skin. Skin laxity can be treated with surgical lifts, which remove some skin, or newer radiofrequency therapies. Renuvion improves skin laxity by improving the shape of the underlying tissues through minimally invasive techniques (i.e. coagulation/contraction of subcutaneous tissues). Refer to the Instructions for Use for the full indications for use of the Renuvion APR and Dermal Handpieces.(https://www.americanboardcosmeticsurgery.org/the-abcs-of-cosmetic-surgery-body-contouring-terms-to-know/)
5. Body contouring, or body sculpting, refers to surgical procedures that improve the appearance of skin and tissue after weight loss. Refer to the Instructions for Use for the full indications for use of the Renuvion APR Handpiece.(https://www.hopkinsmedicine.org/health/treatment-tests-and-therapies/body-contouring#:~:text=Body%20contouring%2C%20or%20body%20sculpting,tissue%20after%20major%20weight%20loss.)
6. Body contouring is a medical or surgical procedure that aims to reshape an area of the body. It may involve procedures that: Get rid of extra skin, eliminate excess fat, reshape or contour the area. Refer to the Instructions for Use for the full indications for use of the Renuvion APR Handpiece.(https://my.clevelandclinic.org/health/treatments/21525-body-countouring)
7. Surgical body contouring following weight loss addresses excess sagging skin and fat while improving the shape of the underlying support tissue. The result is a better-proportioned appearance with smoother contours. Refer to the Instructions for Use for the full indications for use of the Renuvion APR Handpiece. (https://www.plasticsurgery.org/cosmetic-procedures/body-contouring)
